EF-Tu-binding Re-complexed antibiotics - Re-complexed compounds targeting the elongation factor EF-Tu
Ref.-No. 5200
Keywords: Antibiotics, bacteria, infection, Gram-positive
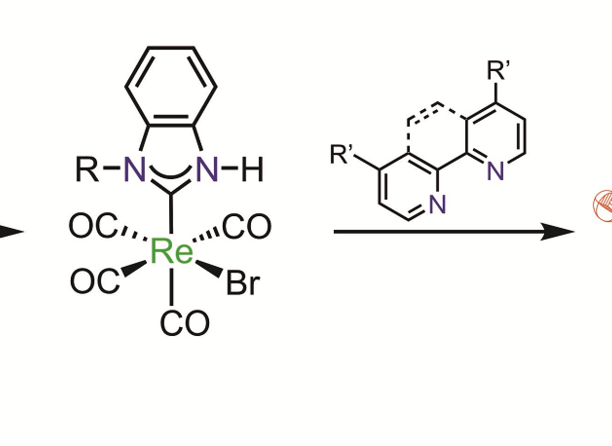
A series of novel Re(I)-(CO)3-N-heterocyclic carbene (NHC) complexes bearing unsubstituted benzimidazol-2-ylidene ligands as well as a variety of bisimine ligands has been prepared and comprehensively characterised. The complexes were found to exhibit potent antimicrobial activity against gram-positive bacterial strains in the low micromolar concentration range, rendering these compounds interesting lead structures for the development of novel metal-based antibiotic agents.
Anti-Gram-positive activity:
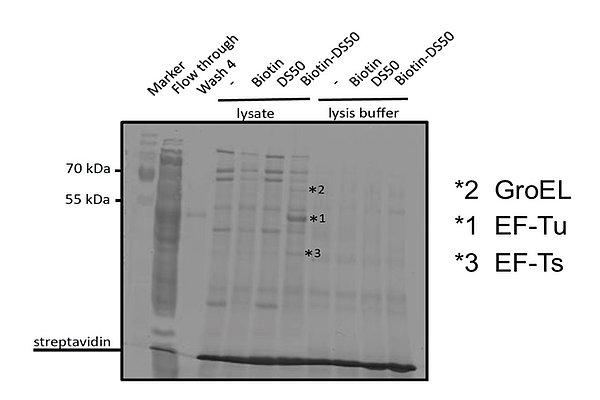
Substances have good activity against Bacillus subtilis and Staphylococcus aureus. Activity against MRSA strains were in the low and sub-micromolar range. No anti-Gram-negative activity was observed. EF-Tu was identified as target in pull-down experiments using biotinylated compound. Protein biosynthesis inhibition was detected in precursor incorporation experiments using unlabeled compound.
Validated target:
Protein biosynthesis inhibition was observed and EF-Tu identified as target protein. Protein biosynthesis is a clinically validated target. Comparative proteomics studies suggest that the mechanism of action is different from known EF-Tu inhibitors.
Competitive Advantages
- Novel antibiotic class
- Activity against Gram-positive Bacteria
- Inhibits translation – a validated target
Commercial Opportunities
The technology is offered for licensing and further therapeutic development.
Current Status
In case of interest we are pleased to inform you about the patent status.
Relevant Puclications
Siegmund, D, et al. (2017) Benzannulated Re(I)-NHC complexes: synthesis, photophysical properties and antimicrobial activity. Dalton Trans. 46: 15269-79.
—
An invention of Ruhr-Universität Bochum.