NMDAR Antagonists as new Antidiabetic Medication - Novel anti-diabetic compounds
Ref.-Nr. 4501
Keywords: Treatment, Diabetes, diabetes type II, NMDA-receptor, pancreatic cells, dextrophan, dextromethorphan
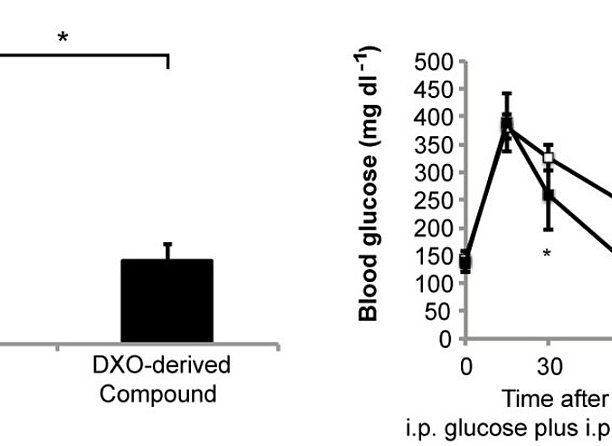
According to the International Diabetes Federation (IDF), diabetes affects close to 400 million people worldwide and caused 500 billion Euros in health expenditure in 2013. Most of the costs are associated with the treatment of subsequent disorders that, to a large extent, are caused by chronic hyperglycaemia. Diabetes is progressive with worsening hyperglycaemia likely caused by progressive decline of pancreatic beta cells, which cannot be stopped by current medication. Reduced blood brain barrier (BBB) permeability of a dextrorphan (DXO)-derived compound compared to DXO (left side). Significant reduction of postprandial blood glucose concentrations upon application of this DXO-derived compound to mice during a glucose tolerance test (right side). In preclinical and clinical trials, the NMDA receptor antagonist dextrorphan (DXO) and its prodrug dextromethorphan have been shown to harbor antidiabetic properties (Marquard et al., Nat Med 2015). In addition, DXO was shown to protect mouse and human pancreatic beta cells from cell death during a diabetogenic setting. DXO is well tolerated and sold as over-the-counter (OTC) medication for more than 50 years. However, adverse events are observed, which are likely caused by the action of DXO on the central nervous system (CNS). Here, the scientists developed DXO-derivatives that do not efficiently pass the blood brain barrier (BBB), and thus should cause fewer adverse effects on the CNS, but maintain their antidiabetic properties. Therefore, the derivatives might maintain the good safety profile and antidiabetic properties of its starting substance dextrorphan, but with fewer adverse effects. The inventors now aim to demonstrate the expected novel clinical benefit in clinical studies. As suggested by recent phase 2a clinical trials using DXO, also the novel compounds may be combined with existing antidiabetic drugs exerting a synergistic effect (Marquard et al., Diabetes Obes Metab 2015).
Vorteile
- Novel approach for reduction of blood sugar levels without risk of Hypoglycaemia
- Preclinical evidence of protection of insulin-secreting beta cells
- Possible prevention or delay of diabetes Progression
- Good results with starting substance in two clinical studies on human type 2 diabetics (Phase IIa)
- Possible enhanced safety and reduced adverse effects compared to the OTC drug dextromethorphan
Kommerzielle Anwendung
On behalf of the Heinrich-Heine-University of Duesseldorf, PROvendis is offering access for commercial use in terms of a license as well as research collaboration to innovative companies.
Aktueller Stand
A priority patent application has been filed for the novel compounds and their use in treating diabetes (patent applications covering the use of DXO for treating diabetes as shown by the inventors laboratory are also pending).
—
Eine Erfindung der HHU Düsseldorf.