Monitoring of hemophilia - Utilizing PD1 as a biomarker for monitoring immune tolerance of coagulation factor VIII treatment of hemophilia A patients
Ref.-Nr. 5490
Keywords: Hemophilia A, factor VIII, monitoring of treatment
Hemophilia A is an X chromosome-linked inherited bleeding disorder with an incidence of 1 in 5,000 male births. It results from mutations of the F8-gene coding for coagulation factor VIII (FVIII) causing low plasma levels or activity of the FVIII protein. Patients with hemophilia A suffer from lifelong bleeding tendencies including spontaneous or traumatic bleeding episodes. Most patients receive protein replacement therapy with plasma-derived or recombinant FVIII, administered by intravenous infusion. The main complication regarding this therapy is the formation of neutralizing anti-FVIII antibodies that occurs in approximately 30% of patients with severe hemophilia A. Such antibodies neutralize the infused FVIII protein, leading to increased hemophilia A morbidity and mortality. To overcome this complication, the standard approach to induce FVIII-specific tolerance is the so-called immune tolerance induction therapy (ITI). ITI involves repetitive injections of high doses of FVIII with or without bypassing agents. This approach is reminiscent of an immunological phenomenon described almost 50 years ago termed “high-zone tolerance”, which denotes the empiric observation that repetitive application of large antigen doses often induces immune tolerance. However, approximately 20-40% of patients undergoing ITI do not achieve long-lasting peripheral tolerance.
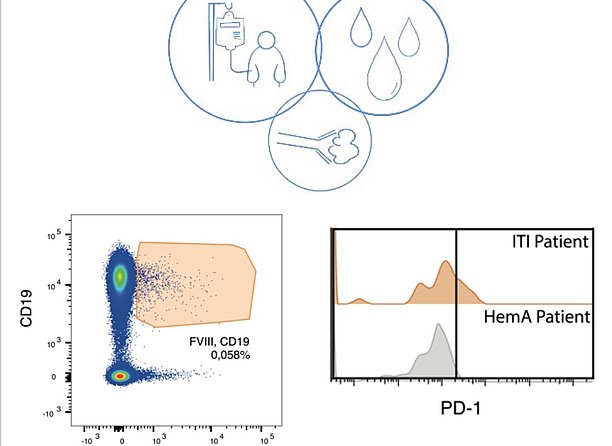
The invention provides a biomarker that allows continuous monitoring of the patients‘ responses to the aforementioned therapy. It also serves as a risk assessment tool to monitor FVIII inhibitor development.
Vorteile
- Novel biomarker for development of neutralizing anti-drug antibodies
- Biomarker can be measured in an easy and objective way
- Enables continuous monitoring of therapeutic response
- Allows risk assessment for failure of immune tolerance induction treatment
Kommerzielle Anwendung
There are more than 240,000 hemophilia A patients worldwide (Annual global survey, world federation of hemophilia, 2020). The global FVIII market is estimated to grow to USD 11 billion in 2021 (Global Factor VIII Deficiency Treatment Market 2017-2021, Technavio). Due to the development of alloantibodies against FVIII, 30% of these patients require immune tolerance therapy. Costs for immune tolerance therapy are estimated at EUR 1 million / year. Hence, the method offered by the invention to closely monitor therapeutic success of FVIII treatment and ITI meets substantial medical and economic needs.
Aktueller Stand
The invention has been validated by studies in mice and men. Blood samples of 31 patients with Hemophilia A and 11 healthy subjects were analyzed. In case of interest, we are pleased to inform you about the current status of the patent.
Technologie-Reifegrad
1
2
3
4
5
6
7
8
9
Versuchsaufbau in Einsatzumgebung
—
An invention of University of Bonn.