Corallopyronin A as antifilarial drug - A natural antibiotic product for the treatment of filariasis
Ref.-Nr. 2838 - human
Keywords: Elephantiasis, River blindness, human, filariasis, symbiont, Wolbachia, human, anthelmintic (anti-worm), anti-filarial, DZIF (Deutsches Zentrum für Infektionsforschung)
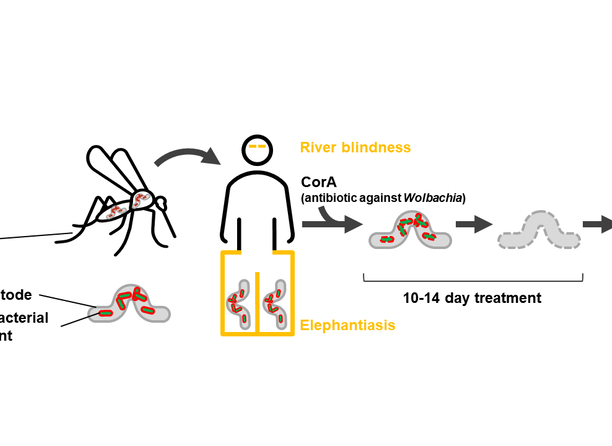
Filariasis is a parasitic disease caused by filarial nematodes that are transmitted to humans through blood sucking mosquitoes. There is a symbiotic relationship between the nematodes and the bacterial endosymbiont Wolbachia that makes the nematodes' life cycle heavily dependent on Wolbachia’s presence. Standard therapeutic treatment for filariasis usually involves drug therapy with antiparasitic drugs or the antibiotic doxycycline. However, said treatment has disadvantages such as long therapeutic treatment, and contraindication for children < 8 years and during pregnancy/breast feeding.
Corallopyronin A (CorA) is a non-competitive inhibitor of the bacterial DNA-dependent RNA polymerase. Wolbachia can efficiently be removed by only one or two treatment regimens of CorA. In addition, side effects were not realised during the pre-clinical studies. A production process of CorA is established on a 15,000 litre scale in preparation for GMP manufacturing. A stable formulation has been developed for oral administration and medical use in logistically difficult regions.
Vorteile
- Effective treatment of filariasis presumably also for children
- Alternative mode of action of CorA avoids selection for rifampicin cross-resistant M. tuberculosis
- Oral administration of CorA
- Stable formulation of CorA for medical care in logistically difficult regions
Kommerzielle Anwendung
On behalf of the University of Bonn, PROvendis offers an access to rights for commercial use (patent applications, patents and know-how).
Aktueller Stand
Assessment CorA | Results |
Production and formulation
|
Each up to 15,000 L 35 to 90 g batches Gastro resistant capsules for oral application |
Toxicology (non-GLP)
|
EC50 [µM]: A3= 11, PPARγ= 2.2, COX1= 8.2 No inhibiton of six recombinant human CYPs EC50 [µM]: 12 |
Safety Pharmacology | ✓ On request |
Pharmacokinetic results | ✓ On request |
Regulatory status
|
✓ 2018 ✓ (Q3/ 2023) Drug product quality, Dose calculation, Phase I trial protocol (Q1/ 2024) |
Technologie-Reifegrad
1
2
3
4
5
6
7
8
9
Versuchsaufbau in Einsatzumgebung
Relevante Veröffentlichungen
Rox et al., Pharmaceutics, 2023; 15(1):131; Ehrens et al., Front. Trop. Dis., Vol. 3 (2022); Krome et al., Nat Prod Rep 39:1705-1720; Krome et al., Pharmaceutics 12 (2020):1105; Becker et al. Pharmaceutics (2022) 14:1657
—
An invention of the University of Bonn.