Extracorporeal apheresis to treat preeclampsia - A novel VEGF-multimer based approach
Ref.-Nr. 5797
Keywords: Treatment, preclampsia, therapy, VEGF-R1 (sFlt-1), VEGF-R1 soluble, apharesis, angiogenic balance, angiogenesis, pregnancy, recombinant VEGF, elimination VEGF-R1, release VEGF and PIGF, PIGF, vascular endothelial growth factor, placental growth factor, uterus, placenta
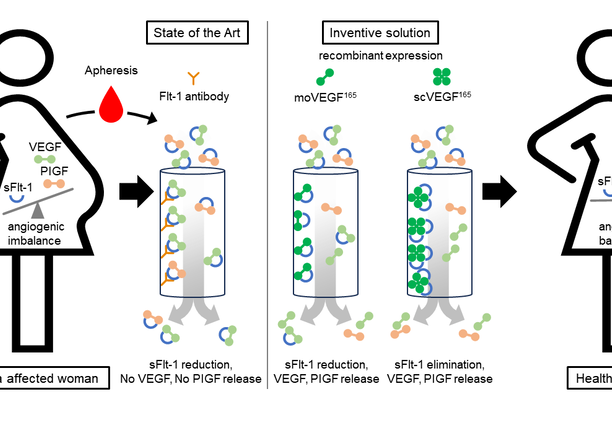
Preeclampsia is still a threat to pregnant women’s health world-wide and in industrialized countries the most common cause of prematurity, including all potential complications of preterm delivery and its sequelae later in life. In the pathogenesis of preeclampsia, increased expression of soluble FMS-like tyosine kinase 1 (sFlt-1) acts as an antagonist by scavenging and neutralizing the pro-angiogenic vascular endothelial growth factor (VEGF) and placental growth factor (PIGF). The resulting angiogenic imbalance may progress to enothelial dysfunction and thrombotic microangiopathy, manifesting with seizures, stroke, or multiorgan failure. Effective therapeutic and prophylactic measures are lacking, limiting interventional options to premature termination of pregnancy. Thus, therapies for preeclampsia are still an unmet and urging medical need.
The underlying invention aims at using an apheresis based approach with recombinant monomeric (moVEGF165)or two single-chain dimers of VEGF165 (scVEGF165). Both compounds enhance binding affinity to sFlt-1 and efficiently reduce or eliminate sFlt-1 levels from plasma of preclampsia affected women. Compared to approaches based on antibodies in the art, the use of both compoundsas a ligand has the additional effect that bound VEGF and PIGF are displaced and released from sFlt-1. Overall, this minimizes the risk of life-threatening conditions and, in particular, restores uterine vascularization and establishment of uteroplacental circulation.
Vorteile
- Restoring the angiogenic balance by displacing endogenous VEGF and PlGF from its binding to sFlt-1
- moVEGF165 and scVEGF165 have a higher affinity to sFlt-1 as compared to antibodies
- Efficient elemination of sFlt-1 from plasma by moVEGF165
- and scVEGF165
Kommerzielle Anwendung
On behalf of the University Hospital of Cologne, PROvendis offers an access to rights for product development and commercial use of this invention with moVEGF165 andscVEGF165.
Aktueller Stand
Before starting clinical studies in pregnancies with preeclampsia, toxicity and compatibility, e.g. the safety of endogenous VEGF- and PlGF-release for mother and fetus, has to be tested in vivo.
Technologie-Reifegrad
1
2
3
4
5
6
7
8
9
Nachweis der Funktionstüchtigkeit
Relevante Veröffentlichungen
Matin, M. et al. (2020) Hypertension. 2020 Oct;76(4):1176-1184. WO2021/185996 A1
—
An invention of University of Cologne.