EasyDMEK Shooter and PrepLoad Tool - Novel tool for controlled unfolding and atraumatic positioning of the Descemet membrane (DC) & device for contactless preparation and transfer of DCs
Ref.-Nr. 4635 / 5542 / 5655
Keywords: corneal transplantation, Descemet´s membrane, Descemet Membrane Endothelial Keratoplasty (DMEK), transplant, DMEK injection device, injector, graft, DMEK tube, eye surgery, ophthalmology, corneal clouding, Device for the preparation of a Descemet’s membrane-endothelium graft, graft transport device
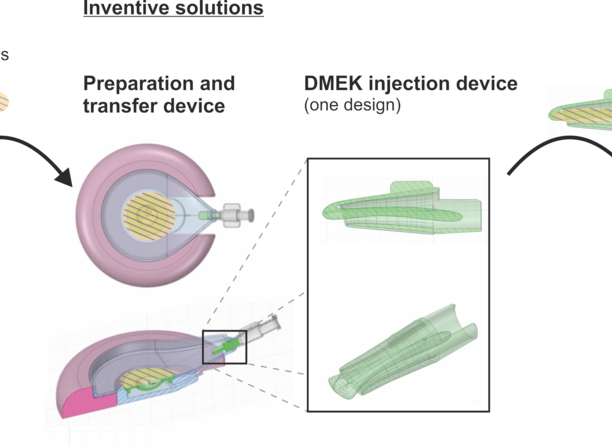
Corneal transplantation is the most successful organ transplantation in medicine and performed on patients with endothelial dysfunction. In a surgery procedure called DMEK (Descemet Membrane Endothelial Keratoplasty), the inner layer of the cornea, the Descemet's membrane, is replaced with a donor membrane. Routinely, lens injectors are used during this procedure to insert the donor membrane into the recipient´s eye precisely. However, the Descemet´s membrane adversely tends to wind itself up to form a single-axis winding. This behaviour is additionally promoted by unsuitable storage/transport containers for the graft. Therefore, the membrane must be uncoiled manually by the surgeon after insertion into the front chamber of the eye, partly with the aid of further instruments and/or by air bubble on the cornea.
Ophthalmologists at the University Hospital of Cologne have developed a DMEK injection device with a special design, which simplifies the release and unfolding of the membrane into the recipient´s eye during surgery. Therefore, the number of subsequent surgical steps and the likelihood of complications is reduced and consequently the success rate of this surgical method is increased.
A specially developed device allows the Descemet membrane to be prepared and transferred into an injection cartridge without contact. This procedure reduces the loss of the endothelial cells of the transplant (descement membrane with endothelium).
Vorteile
- DMEK injection device: controlled unfolding of the Descemet membrane, Reduced risk of complications, Increased success rate
- Preparation and storage device: No contact with the graft → reduction in the loss of endothelial cells
Kommerzielle Anwendung
On behalf of the University Hospital of Cologne, PROvendis offers an access to rights for commercial use of this invention.
Aktueller Stand
The DMEK injection device was manufactured using 3D printing on a plastic basis.
Technologie-Reifegrad
1
2
3
4
5
6
7
8
9
Nachweis der Funktionstüchtigkeit
Relevante Veröffentlichungen
Siebelmann et al. Cornea 2020 May;39(5):605-608.
EP3474776 B1, US 10,874,504, PCT/EP2020/055687, PCT/EP2020/072701
—
An invention of the University of Cologne.