A Multifunctional Linker for CAR T-cells
Ref.-Nr. 4813
Keywords: CAR T cells, immunotherapy, purification, CD34, hinge-region
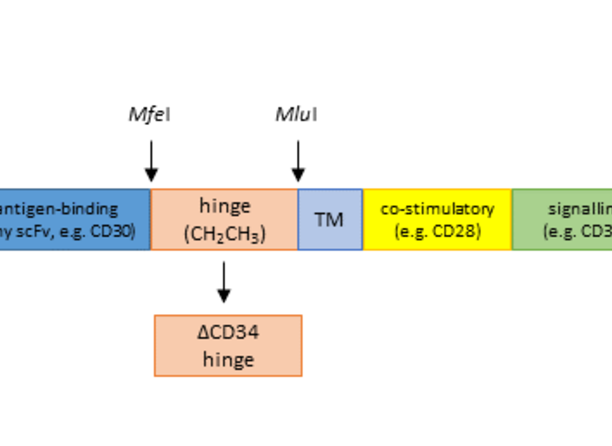
Chimeric antigen receptors (CARs) have been developed for the molecular engineering of effector T-cells to be used in targeted cancer therapy. CARs consist of the antigen-binding single-chain fragment (scFv) of an antibody fused via a hinge region to a transmembrane domain (TM) and to one or more intracellular signalling co-stimulatory regions. CAR expressing T-cells are now able to specifically and in an MHC-independent manner recognize the corresponding tumor-associated Antigens. The different scFv-regions determine the specifity and affinity of the CARs. Hundreds of different CARs have been generated with the clinically most successful so far being CAR constructs recognizing the B-cell-associated antigen CD19 on leukemias and lymphomas. The hinge region in CARs functions as a flexible spacer for the scFvs and improves the recognition/function of CARs for antigens closer located to the surface of target cells. For a large number of constructs, the hinge region consists of the CH2CH3 domain of a human IgG antibody, which can bindsto Fcg-receptors on macrophages and other cells thereby resulting in cross-activation and activation-induced cell death in vivo independent of recognition of the target antigen. The researchers in this invention haved replaced the CH2CH3 site by fragments of the human CD34 antigen that contain the epitope for the CD34 antibody QBEND10 that is used in the CliniMACS device sold by Miltenyi Biotech GmbH. This exchange has decisive benefits: first, unwanted immune side-effects are potentially prevented. Second, modified T cells can be easily detected using standard flow cytometry; thus engraftment and persistence of transduced T-cells in vivo can be readily assessed. Ultimately, using this hinge domain, modified T-cells can easily and rapidly be selected and enriched by the CliniMACS system for clinical use. By including this hinge region in CAR constructs for selection, common viral and also nonviral vectors for CAR transfer have sufficient space for the introduction of a safety switch (i.e. a suicide gene such iCasp9), that facilitates the use of allogeneic or even haploidential donor effector cells.
Vorteile
- Rapid and easy staining for follow-up of CARs
- Rapid and easy isolation of successfully engineered CAR T cells
- Not susceptible to Fcg‑receptor binding
- Modular composition enables other modifications in the hinge region
Kommerzielle Anwendung
The technology is offered for licensing and further therapeutic development.
Aktueller Stand
The researcher are preparing mice studies to confirm in vivo efficacy. In case of interest we are pleased to inform you about the patent status.
Relevante Veröffentlichungen
Roellecke, K., et al. (2016) Gene Therapy 23: 615-26.
—
Eine Erfindung der Universität Münster und HHU Düsseldorf.